How Drugs Affect pH and Alkalinity in the Body and Blood
When we talk about the health of our body and its various systems, one of the most critical factors to consider is its pH balance and alkalinity. Drugs, whether prescription medications, recreational substances or even supplements, can significantly influence the pH levels and the overall alkalinity of the body and blood. Maintaining the right balance is essential for optimal function, and any deviation can lead to serious health consequences. At Bluff in Augusta, GA, we understand how important it is to be aware of these interactions for those seeking to live a healthier lifestyle. Here, we dive into how drugs can alter the pH balance of your body.
What is pH and Alkalinity?
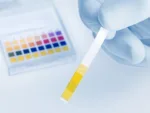
pH is a scale that measures how acidic or alkaline (basic) a substance is, ranging from zero to 14. A pH of seven is considered neutral, values below seven indicate acidity and values above seven represent alkalinity. The body works to maintain a slightly alkaline pH, typically around 7.35 to 7.45 in the blood, which is crucial for cellular function, enzyme activity and overall metabolic processes.
Alkalinity refers to the body’s ability to neutralize acid. The body employs a complex buffering system, including organs like the kidneys and lungs, to regulate pH levels. Maintaining a proper pH balance is vital; even a small shift can result in significant physiological effects.
How Drugs Affect pH and Alkalinity
- Acid-Forming Drugs
Many substances, including certain medications, can lower the body’s pH, making it more acidic. This is especially true for substances such as alcohol, caffeine, nicotine and even some prescription medications. For instance, nonsteroidal anti-inflammatory drugs (NSAIDs), like ibuprofen, can increase acid production in the stomach, which can, in turn, affect blood pH by creating an acidic environment.
Recreational drugs such as cocaine or methamphetamine can also disturb the body’s natural pH balance. These drugs are known to elevate cortisol levels, a stress hormone that leads to an increased production of acids in the body. As a result, chronic use of these substances can contribute to acidosis, a condition where the body becomes too acidic, causing symptoms like fatigue, difficulty breathing and confusion.
- Alkaline-Forming Drugs
On the other side of the spectrum, some medications and substances can promote alkalinity. For example, certain antacids used to treat acid reflux, such as calcium carbonate or sodium bicarbonate, work by neutralizing excess stomach acid. While this action is effective in the short term for digestive discomfort, it can influence the body’s overall alkalinity, particularly in the urine.
Intravenous (IV) fluids used in hospitals often contain bicarbonate to treat acidosis. These fluids are designed to help raise the blood’s pH, moving it back into a more alkaline state. In some cases, alkalizing agents are prescribed for patients with kidney disease or other conditions that cause the body to retain too much acid. This can help prevent complications like kidney stones and muscle weakness.
- The Role of the Kidneys and Lungs in pH Regulation
The kidneys and lungs play an essential role in regulating the body’s pH. Drugs can directly impact these organs’ ability to maintain pH balance. For example, diuretics are commonly used to treat conditions such as high blood pressure or fluid retention, but they can alter the levels of electrolytes, including bicarbonate, which in turn affects pH. Diuretics can either lead to acidosis or alkalosis, depending on the type of drug and how it affects electrolyte balance.
Similarly, substances like opiates or sedatives can slow down respiratory rate, which reduces the body’s ability to expel carbon dioxide. Since carbon dioxide reacts with water in the body to form carbonic acid, a reduction in respiratory rate can lead to an increase in acidity, disrupting the body’s pH and leading to respiratory acidosis.
- Long-Term Effects of Drug Use on pH Balance
Chronic drug use can have long-lasting effects on pH balance. For example, prolonged use of stimulant drugs can lead to sustained low pH levels in the blood, causing a condition known as metabolic acidosis. Over time, this can strain the kidneys and lungs, both of which are involved in regulating pH levels, leading to a cascade of health problems like kidney failure or impaired lung function.
Additionally, drug addiction can lead to poor diet and lifestyle choices, which can further disrupt the body’s pH balance. A diet rich in processed foods and low in fruits and vegetables can contribute to acidity in the body, while dehydration can also prevent the kidneys from efficiently removing excess acid.
The Importance of Monitoring pH Levels
For those who are on medication or use recreational drugs, it’s important to monitor pH levels regularly. Regular blood tests can help identify early signs of imbalances in pH or alkalinity, allowing for prompt intervention. If you are concerned about how your medications or lifestyle choices might be affecting your body’s pH balance, it’s always a good idea to consult with a healthcare provider.
Drugs play a significant role in regulating or disrupting the pH levels and alkalinity in the body. Whether they are acid-forming or alkalizing, their impact can be profound. By understanding how drugs influence the pH balance and taking steps to manage it, individuals can support their overall health and ensure their body remains in optimal condition. At Bluff Augusta, we are committed to providing resources and support for maintaining a healthy lifestyle, so you can make informed decisions about your health.